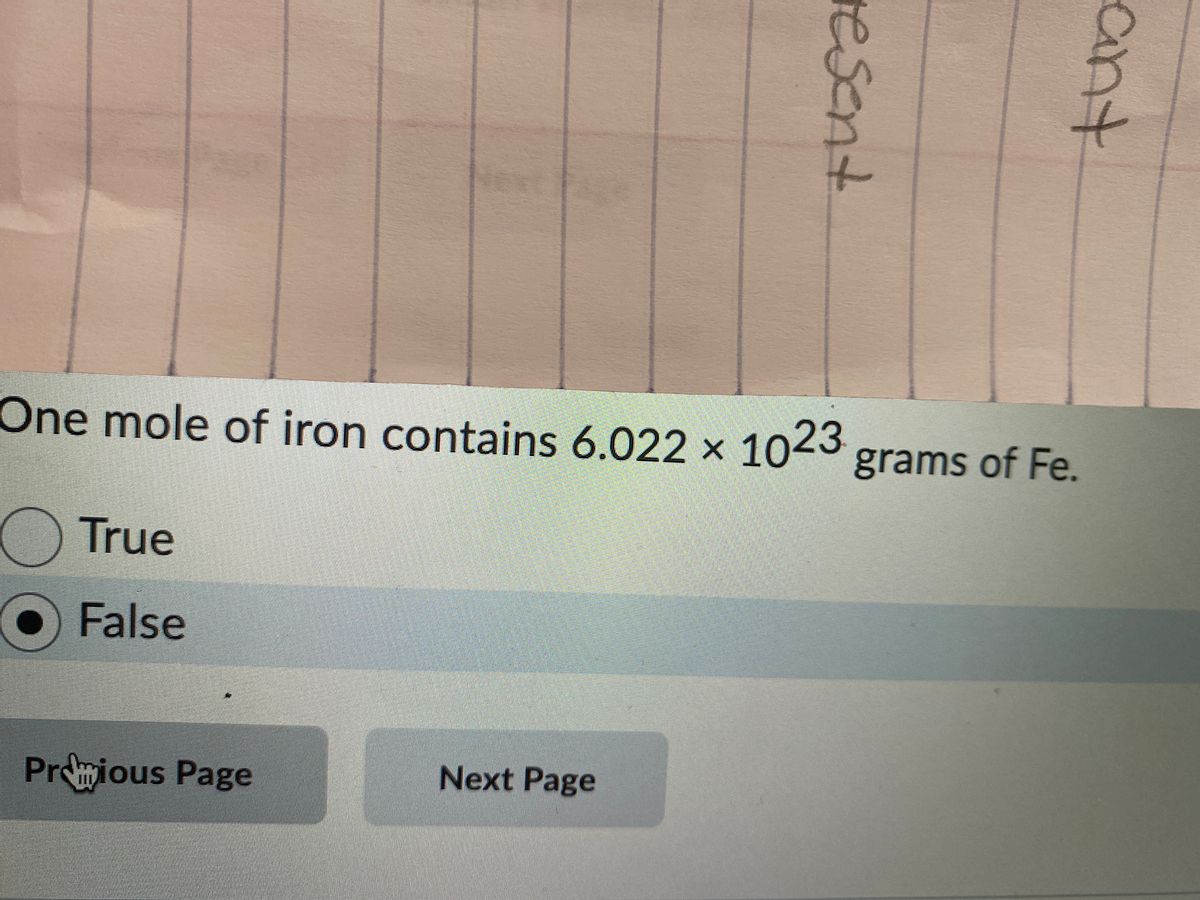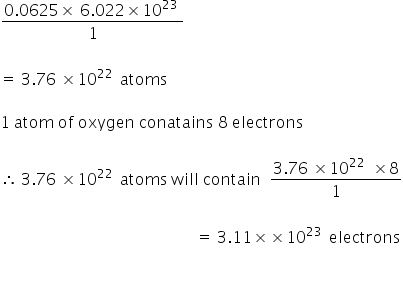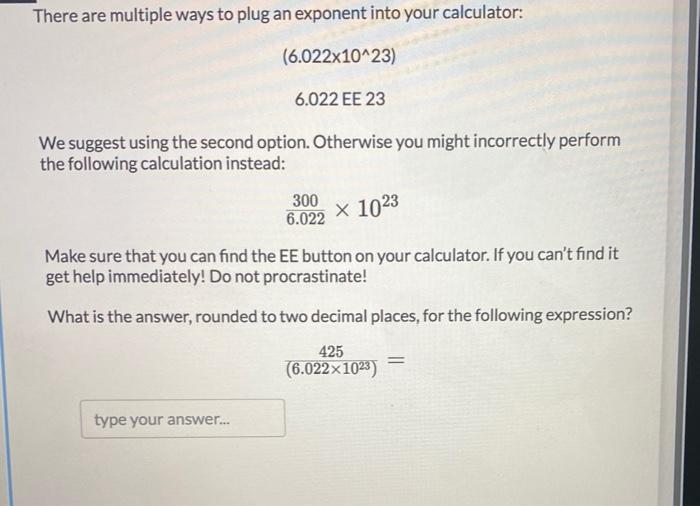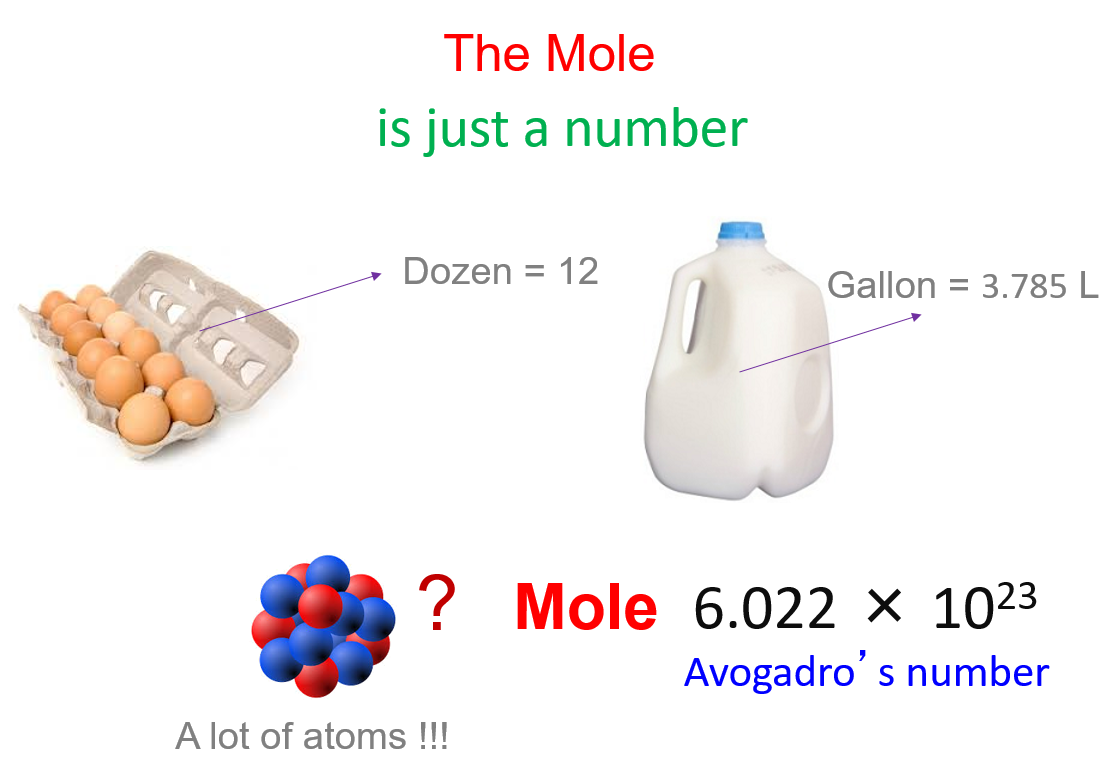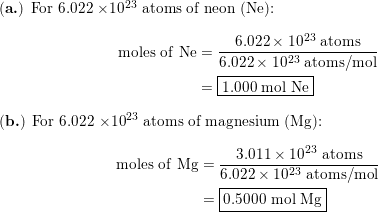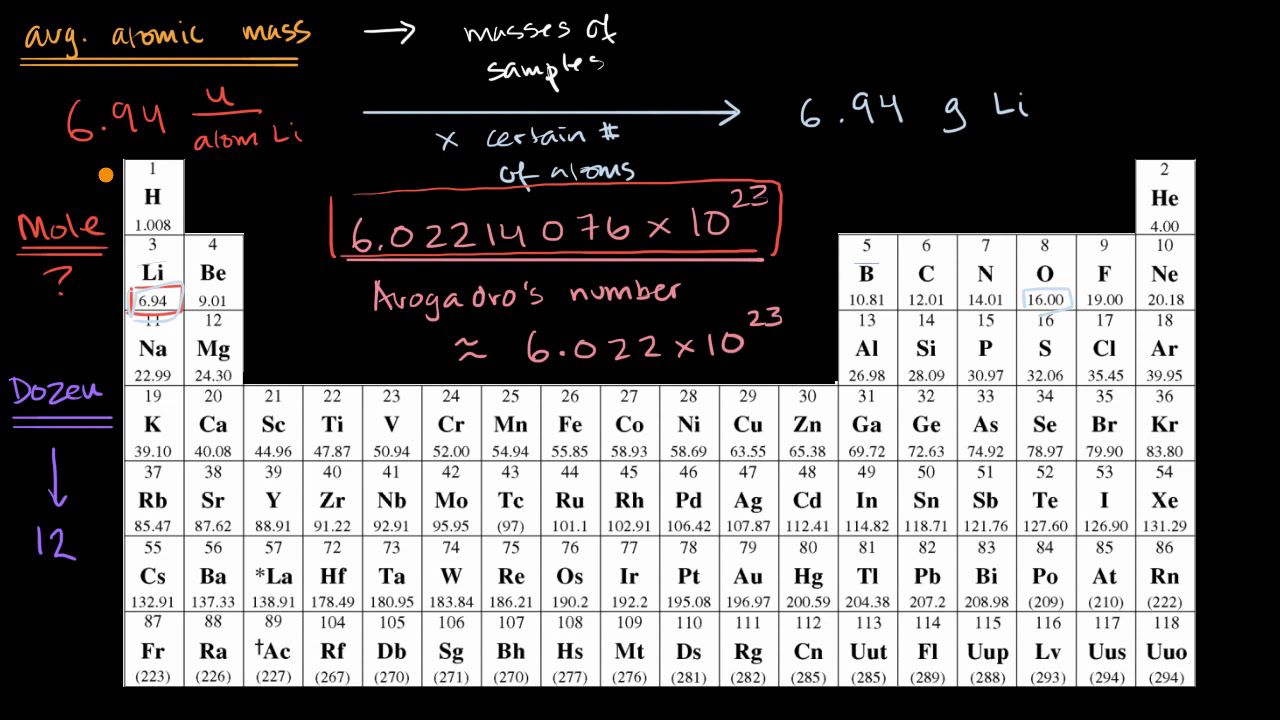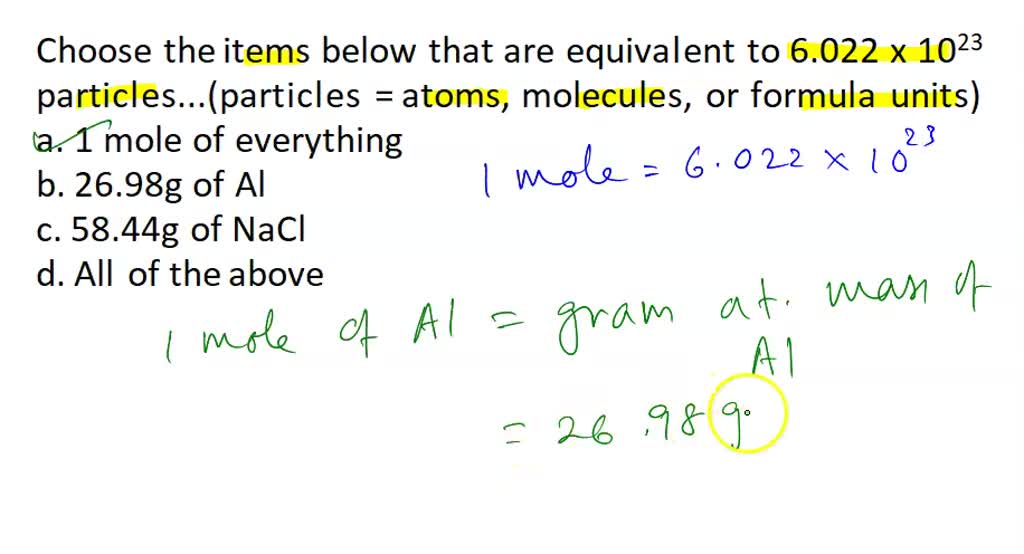
SOLVED: Choose the items below that are equivalent to 6.022 x 1023 particles...(particles = atoms, molecules, or formula units) a. 1 mole of everything b. 26.98g of Al c. 58.44g of NaCl

Question Video: Determining the Number of Oxygen Atoms Present in a Given Number of Moles of Aluminum Nitrate | Nagwa

1 mole = 6 022 x 10^23 If there is 1 mole of H2 we have multiply the Avogadro no - Science - Atoms and Molecules - 15776529 | Meritnation.com

Calculate the mass of 6 022 x 10^23 molecules of CaCO3 - Science - Atoms and Molecules - 13283691 | Meritnation.com

Oh My!!. Mole (mol) can be defined as the number equal to the number of carbon atoms in grams of carbon (in an chemical equation it is the coefficients. - ppt download